EstroG-100® (a blend of Cynanchi Wilfordii Radix and other complex extracts) is the first functional ingredient for women's menopause recognized by the Korean Ministry of Food and Drug Safety. It is a blend of Cynanchi Wilfordii Radix, Phlomis Umbrosa Turczaninow, and Angelica Gigas.
Through numerous clinical trials and research papers, its effectiveness in improving ten menopause symptoms has been confirmed. Notably, it does not affect estrogen levels (E2, FSH) and does not bind to estrogen receptors, ensuring it is safely consumed without risk of exacerbating women’s health issues.
Additionally, EstroG-100® has received global recognition, including NDI certification from the U.S. FDA, NPN licensing from Health Canada, and approval from the European Food Safety Authority as a novel food. It is cherished by many middle-aged and elderly women worldwide.

Exclusivity
| Country | registration number | Country(s) | registration number |
|---|---|---|---|
| South Korea | 10-1141194 | Europe (Germany, UK, France, Spain, Italy, Poland) | 2370072 |
| The United States | 9,433,595 | ||
| Canada | 2745020 | Mexico | 328059 |
| Russia | 2491926 | Malaysia | MY-184493-A |
| China | ZL200880132506.0 | Indonesia | ID.P.000050866 |
| Australia | 2008365666 | Brazil | PI0823391-8 |
Approval
| Country | Permit name |
|---|---|
| The United States | New Dietary Ingredient |
| Canada | Natural Health Product |
| Europe | Novel Food Ingredient |
| Japan | Food and Drug Classification Search |
| Malaysia | Food Supplement, Traditional Medicine |
| The Philippines | Herbal Medicine |
| India | Food for Special Dietary Use |
| Iran | Natural Product |
| Egypt | Herbal Medicine Product |
Mechanism of Action
No change in weight, BMI and liver enzymes
No change in serum E2 & FSH
No influence on estrogen-sensitive tissues
No binding affinities for ERα, ERβ, and nonselective ER
No toxic effects shown in toxicity studies
5 Human clinical studies published
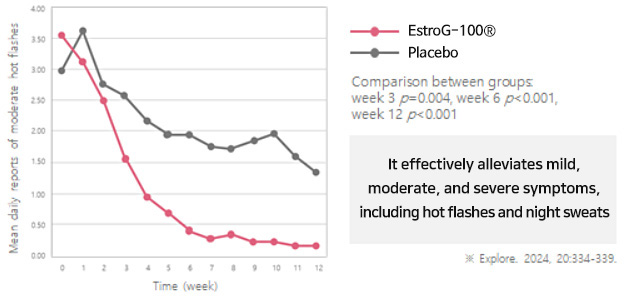
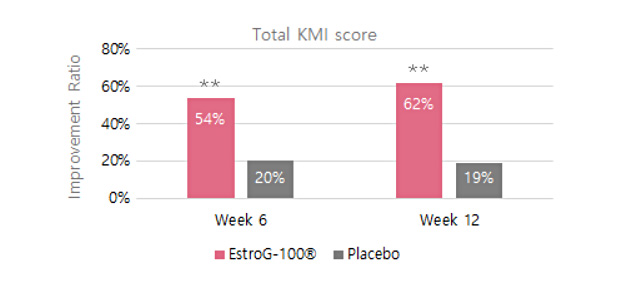 KMI(Kupperman menopause index, 갱년기지수) Improved by 62%
KMI(Kupperman menopause index, 갱년기지수) Improved by 62% 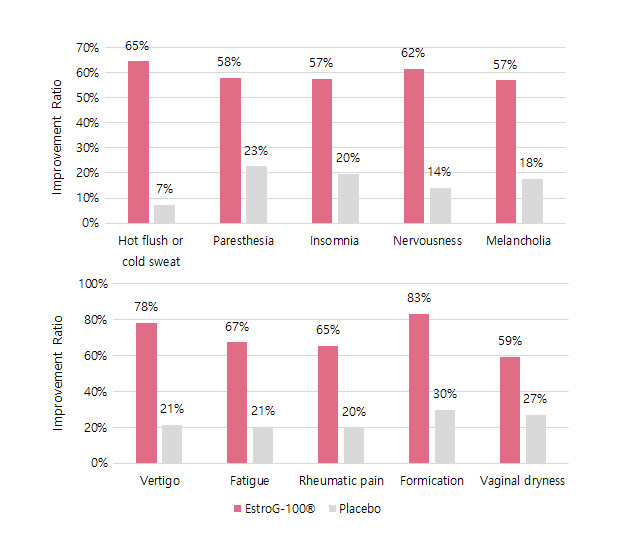 Improves 10 menopausal symptoms(Facial flushing and sweating, numbness in hands and
Improves 10 menopausal symptoms(Facial flushing and sweating, numbness in hands andfeet, insomnia, nervousness, depression, dizziness, fatigue, joint and
muscle pain, feeling cold, and vaginal dryness.)※ Phytother. Res. 2012, 26:510-6.
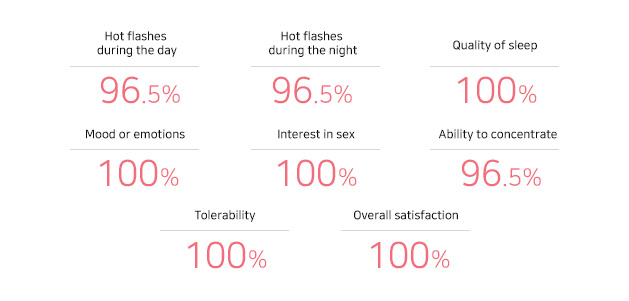
EstroG-100® Satisfaction Improvement
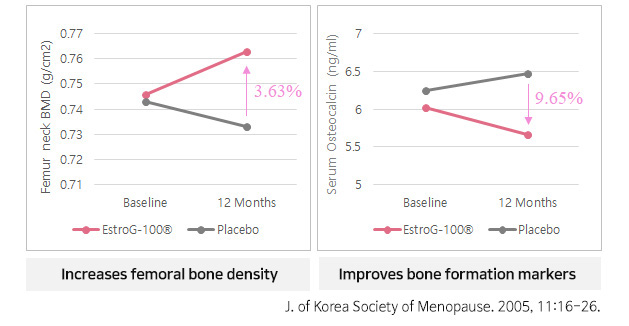
Numerous in vitro & in vivo studies
- Tail skin temperature (TST) ↓
- Femoral bone mineral density ↑
- Sleep induction time ↓
- Sleep continuity time ↑
- Recovery time ↓
- Melatonin in plasma & GABA in brain ↑
- Behavior evaluation ↑
- Serotonin & dopamine in brain↑
- Corticosterone in serum ↑
- Edema rate↓
- Cartilage maintenance factors (Col2α1, Aggrecan, Proteoglycan, SOX-9, Collagen I, Collage X) ↑
- Cartilage destruction factors (TIMP-1, MMP3, MMP7) ↓
- Degenerative osteoarthritis inducing factors (COX-2, MMP, NF-κB, TNF-α, IL-1β) ↓
- Glycogen content in muscle ↑
- LDH activity in muscle ↓
- Genetic expression of exercise fatigue recovery factors (PPAR-δ, UCP-3) ↑
- Antioxidant enzyme activity (SOD, CAT, GST) ↑
- Antioxidant compounds in liver (GSH) ↑
- Oxidative stress marker in liver (MDA) ↓








