ME.NO.PAUSE.
EstroG-100®
EstroG-100®(백수오 등 복합추출물)은 백수오, 한속단, 당귀를 배합한 국내 최초 식약처 여성 갱년기 기능성 인정 원료입니다.
다수의 인체적용시험 및 연구논문을 통해 10가지 갱년기 증상 개선 효과를 확인하였으며
특히 에스트로겐 수치(E2, FSH)에 영향이 없고, 에스트로겐 수용체에 결합하지 않아 여성 질환으로부터 안전하게 섭취할 수 있습니다.
또한 미국 FDA 기능성 물질(NDI) 인증, 캐나다 식약청 천연•기능성 물질(NPN) 라이선스 획득, 유럽식품안전청 노블푸드 통과 등을 통해
전 세계적으로 많은 중년 및 노년기 여성들의 사랑을 받고 있습니다.

Exclusivity
글로벌 특허 획득 현황
특허 명칭: 폐경기 증상의 예방 또는 치료용 식물성 에스트로겐 조성물
(Phytoestrogenic Compositions for Preventing or Treating Symptoms Associated with Menopause)
(Phytoestrogenic Compositions for Preventing or Treating Symptoms Associated with Menopause)
| 국가 | 등록번호 | 국가 | 등록번호 |
|---|---|---|---|
| 대한민국 | 10-1141194 | 유럽(독일, 영국, 프랑스, 스페인, 이탈리아, 폴란드) | 2370072 |
| 미국 | 9,433,595 | ||
| 캐나다 | 2745020 | 멕시코 | 328059 |
| 러시아 | 2491926 | 말레이시아 | MY-184493-A |
| 중국 | ZL200880132506.0 | 인도네시아 | ID.P.000050866 |
| 호주 | 2008365666 | 브라질 | PI0823391-8 |
Approval
| 국가 | 인허가명 |
|---|---|
| 미국 | New Dietary Ingredient |
| 캐나다 | Natural Health Product |
| 유럽 | Novel Food Ingredient |
| 일본 | 식약구분조회 |
| 말레이시아 | Food Supplement, Traditional Medicine |
| 필리핀 | Herbal Medicine |
| 인도 | Food for Special Dietary Use |
| 이란 | Natural Product |
| 이집트 | Herbal Medicine Product |
Mechanism of Action
에스트로겐 활성으로 인한 부작용 없이 안전하게 10가지 갱년기 증상 개선
No adverse event at all subjects
No change in weight, BMI and liver enzymes
No change in serum E2 & FSH
No influence on estrogen-sensitive tissues
No binding affinities for ERα, ERβ, and nonselective ER
No toxic effects shown in toxicity studies
No change in weight, BMI and liver enzymes
No change in serum E2 & FSH
No influence on estrogen-sensitive tissues
No binding affinities for ERα, ERβ, and nonselective ER
No toxic effects shown in toxicity studies
5 Human clinical studies published
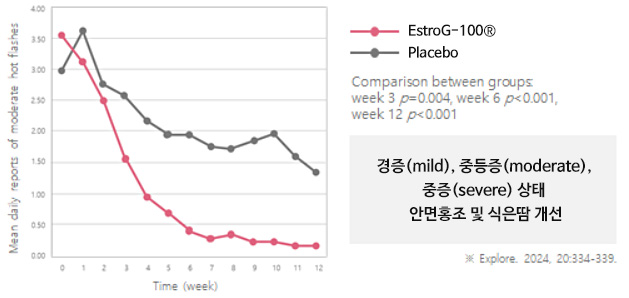
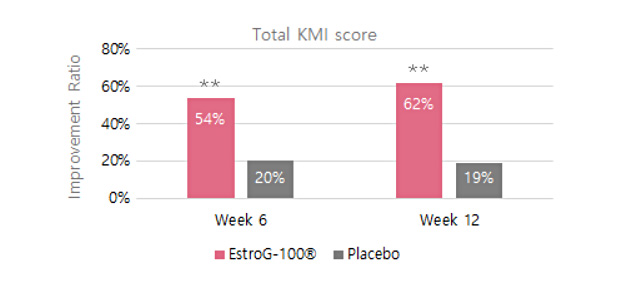 KMI(Kupperman menopause index, 갱년기지수) 62% 개선
KMI(Kupperman menopause index, 갱년기지수) 62% 개선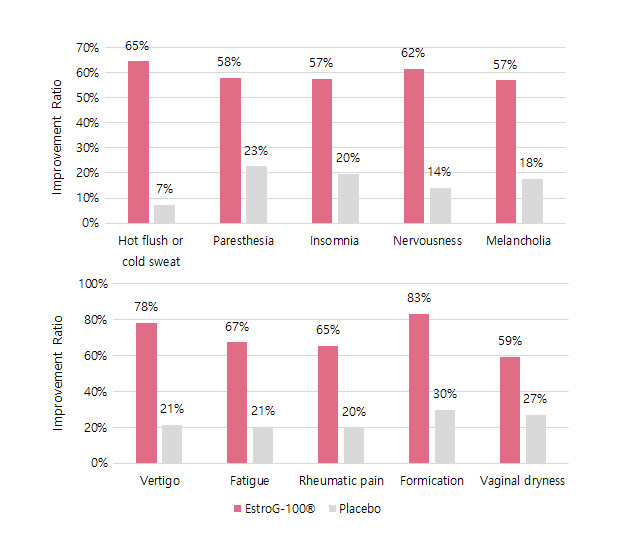 10가지 갱년기 증상 개선(안면홍조 및 발한, 손발저림, 불면증, 신경질, 우울증, 어지럼증, 피로감, 관절 및 근육통, 의주감, 질 건조)※ Phytother. Res. 2012, 26:510-6.
10가지 갱년기 증상 개선(안면홍조 및 발한, 손발저림, 불면증, 신경질, 우울증, 어지럼증, 피로감, 관절 및 근육통, 의주감, 질 건조)※ Phytother. Res. 2012, 26:510-6.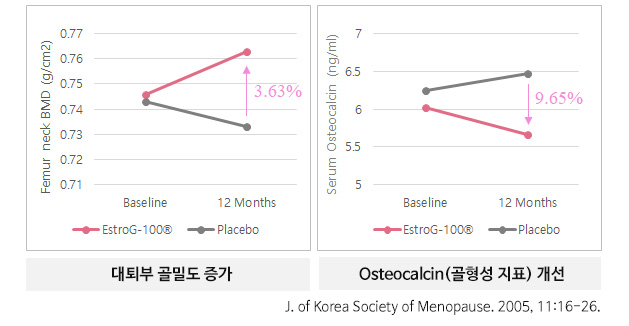
40~70세 여성 60명 대상 12주 섭취
EstroG-100® 섭취 만족도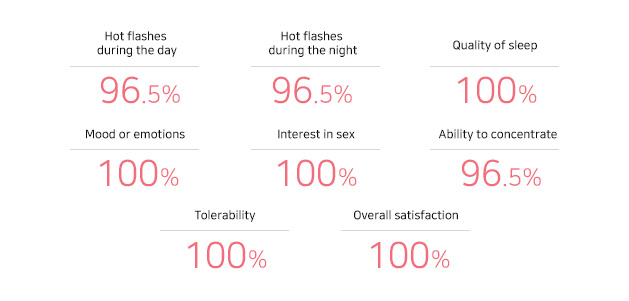
갱년기 증상 개선 전반적 만족도 100%J Midlife Health. 2023, 14:285-290.EstroG-100® 섭취 만족도

Numerous in vitro & in vivo studies
안면홍조
- Tail skin temperature (TST) ↓
골다공증
- Femoral bone mineral density ↑
불면증
- Sleep induction time ↓
- Sleep continuity time ↑
- Recovery time ↓
- Melatonin in plasma & GABA in brain ↑
우울증
- Behavior evaluation ↑
- Serotonin & dopamine in brain↑
- Corticosterone in serum ↑
관절염
- Edema rate↓
- Cartilage maintenance factors (Col2α1, Aggrecan, Proteoglycan, SOX-9, Collagen I, Collage X) ↑
- Cartilage destruction factors (TIMP-1, MMP3, MMP7) ↓
- Degenerative osteoarthritis inducing factors (COX-2, MMP, NF-κB, TNF-α, IL-1β) ↓
피로
- Glycogen content in muscle ↑
- LDH activity in muscle ↓
- Genetic expression of exercise fatigue recovery factors (PPAR-δ, UCP-3) ↑
- Antioxidant enzyme activity (SOD, CAT, GST) ↑
- Antioxidant compounds in liver (GSH) ↑
- Oxidative stress marker in liver (MDA) ↓
Recommended dosage
175 ~ 514 mg/day








